Nerve cells, neurons, are the body’s cosmic messengers. But what transpires when these messengers lose their protective myelin layer? This is the reality for those wrestling with neurodegenerative diseases like multiple sclerosis, where the protective coat, myelin, deteriorates.
Imagine: A 20-year-old debater confidently steps onto the stage, her heart racing. As applause surrounds her, she starts her passionate speech but everything becomes hazy. She lost her balance and crumbled to the ground. The panic in the room is palpable. The diagnosis was one no one saw coming: a neurodegenerative disease. Despite treatments, her once-prominent career fades into a distant memory.
“The brain is the last and grandest biological frontier, the most complex thing we have yet discovered in our universe” ~ James D. Watson, the director of the National Center for Human Genome Research.
While remyelination has been known since around 2007, recent developments and the growing number of neurodegenerative diseases have increased interest in the field. The scientific community is working hard to turn understanding into treatments. With the progress made over the past years, remyelination is now seen as a promising approach against neurodegenerative diseases.
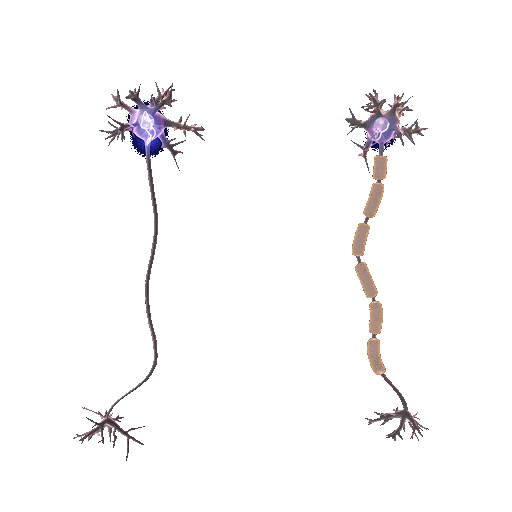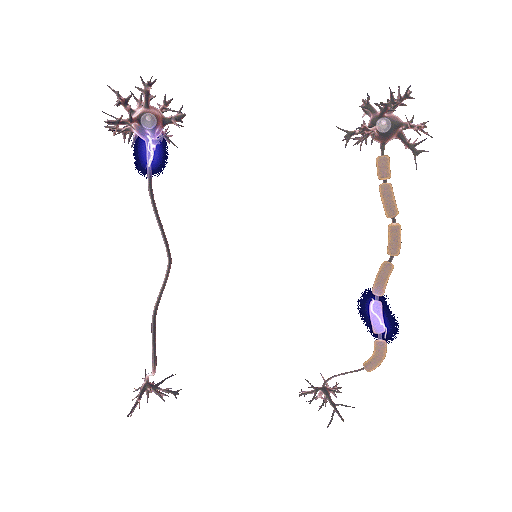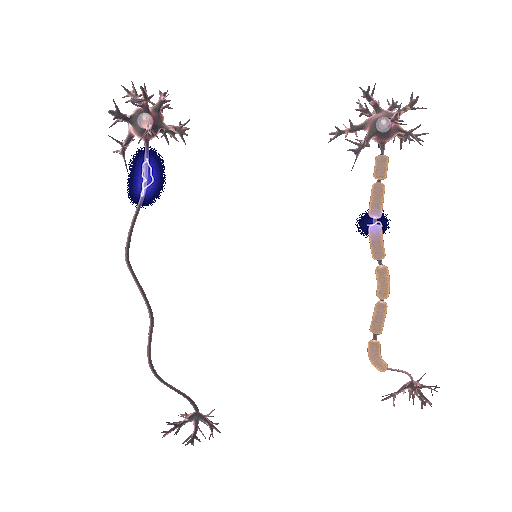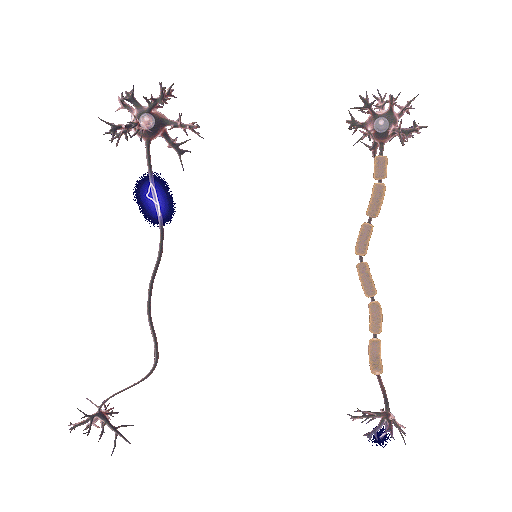
Demyelination involves the deterioration or loss of the myelin sheath enveloping nerve fibers. It is mainly initiated by autoimmune reactions, viral infections, or direct nerve injuries. Myelin, teeming with lipids, expedites electrical impulses along nerve fibers. When this protection is compromised, the speed and precision of these impulses falter, generating neurological symptoms. But why does the protection wear off?
Dr. Wang, an assistant professor of Biotechnology and Bioindustry Sciences at National Cheng Kung University, states that “the “reason” is almost an impossible mission for us right now under certain limitations (technologies, ethic issues…etc).”
In the absence of the myelin sheath, nerve fibers become vulnerable to escalating damage and decay. The precise pathway of demyelination fluctuates based on its origin. For example, in multiple sclerosis, an autoimmune disease, the immune system mistakenly attacks and disintegrates the myelin thinking that myelin is a foreign cell.
Conversely, remyelination is the regeneration of myelin sheaths around previously stripped axons. Oligodendrocyte progenitor cells (OPCs), specific to the central nervous system, are central players. They mature into oligodendrocytes, the very architects of the myelin sheath, initiating repairs when damage occurs. This process doesn’t only restore function but also offers neuroprotection, mitigating further neurological decline.
Unfortunately, remyelination still has a ways to go to address the problem of neurodegeneration For example, Dr. Chih-Yen Wang said, “If simply focusing on “remyelination” or “producing myelin”, I believe we have had a good amount of findings. However, myelin dynamics under pathological conditions is an unsolved issue for treating neurodegenerative diseases. That is, myelin and its producing cells, oligodendrocytes are so sensitive and vulnerable to the disease environment that expected regeneration does not happen or is not sufficient for functional recovery. The gap is still how we can induce oligodendrocyte production, the rate-limiting step for remyelination, in the stressed tissue (e.g. hypoxia and inflammation).” Adding on, Neuroscience Club Leader, Rishi Janakiraman (27’), states, “commonly-administered MS treatments have been focused on acute sites of lesion and relapse prevention, rather than the full progression of the degeneration being reversed.”
Methods and Approaches for Remyelination:
How can we promote remyelination? Here’s how:
Stem Cell Therapy Approach
A trailblazing approach to remyelination involves stem cell therapy. This strategy administers stem cells, which transform into the needed cell type to repair the impaired myelin. Stem cell’s main purpose is to compensate for missing cells in any part of the body. It is a way it helps the body to heal. Stem Cell Research & Therapy says, “Our findings demonstrated that BMSCs promote remyelination in the spinal cord of HD-exposed rats via TNFα/RelB-Hes1 pathway, providing novel insights for evaluating and further exploring the therapeutical effect of BMSCs on demyelinating neurodegenerative disease”(Li, S. et al). Let’s break this down. HD-exposed rats are rats that have Huntington’s Disease which is a demyelinating disease. Testing stem cells on these mice can help determine if it would efficiently work for humans too. The TNFα/RelB-Hes1 pathway is a specific signaling pathway inside the cells. “TNFα”, “RelB”, and “Hes1” are specific proteins or molecules involved in this pathway. In this case, stem cells affected the signaling pathway that involves these molecules.
While stem cell therapy is promising, Dr. Wang brings attention to the challenges, noting, “You may think the ideal way is always the stem cell therapy. However, as you may know, these cells in the lesion end up turning into the supporting cells, astrocytes. It is difficult to induce stem cells/progenitor cells into neurons and oligodendrocytes in the stressed tissue.”
There was an ethical issue too, which is, as Dr. Wang says, “the way we obtain the stem cell. But, as the developing techniques of iPSC [induced pluripotent stem cells], stem cell therapy will be no longer a problem.”
The continuous collaboration between researchers, clinicians, and the broader scientific community is vital to translating these promising laboratory findings into real-world treatments that could change the lives of millions suffering from demyelinating conditions.
Pharmacological Approach
Another method harnesses drugs that push the body’s own cells to reproduce myelin. McMakin from National Institutes of Health states, “Tesar’s team found that two compounds in particular, miconazole (an antifungal) and clobetasol (a steroid), stimulated mouse and human OPCs into generating myelin-producing cells.” Two drugs or medications have activated the oligodendrocytes (cells that produce myelin) to generate more myelin. These drugs target human cells, promoting the body’s cells to restore the sheath itself. .
Janakiraman discusses a potential challenge with this approach, “few studies involve remyelination therapy trials where oligodendroglial precursor cells are recruited to improve axonal function. Stimulating this pathway has immense potential, but it certainly doesn’t get the same share of attention as inflammatory-targeting drugs for MS. One of the main challenges with endogenous remyelination likely involves the inefficient clearance of myelin debris, yet both routes of MS treatment have their respective flaws.”
Despite the challenges, such as complex regulatory hurdles, high research and development costs, and concerns about long-term safety profiles, this approach still has significant benefits. Pharmaceutical research is actively exploring the potential of such compounds to serve as therapeutic agents for demyelinating disorders.
Diet Approach
Dietary habits and physical activity have long been recognized for their profound impact on general health and disease prevention. However, recent studies, such as those from the Mayo Clinic, have delved deeper into the intricate relationships between specific types of diets, exercise, and the health of remyelination.
Janakiraman elaborates, “The benefits of healthy diet and exercise have been empirically supported by neurological data over many years of trials. An intake of foods that reduce cardiovascular risk directly benefits the nervous system — healthy BP and LDL cholesterol levels follow a reduced likelihood of progressive neurodegeneration with age. There have been similar trends with exercise — where more physically active people have a decreased risk of dementia as well as neuroplastic benefits too.”
While a high-fat diet has traditionally been linked to several health issues, in conjunction with regular exercise, it appears to offer benefits in terms of myelin protein production. This relationship suggests that the effects of dietary choices on our neurological health are not solely dictated by the food’s inherent nutritional value but rather by the interaction of our diet with our physical activity levels.
Dr. Wang reveals that many other lifestyle choices can improve neurological health. He says, “To improve neurological health, it is all about how we reduce the chronic stress of our body and increase our brain activity. Inflammation can interfere with proper neuron functioning, thus, impairing our neurological health. Nowadays we can get a lot of information about what can make us inflamed in our body and brain, like excessive fat, injuries, shift working, mental stress, and alcohol.”
To optimize neurological health, a holistic approach considering both diet and lifestyle factors is essential. These findings underscore the importance of personalized health strategies and the need for further research to fully understand and harness the potential of diet-exercise combinations for neurological well-being.
Remyelination is like giving the nervous system a fresh coat of paint, helping it work better. Stem cell therapy, pharmacological interventions, and dietary adjustments each offer unique potential benefits; however, transitioning from laboratory findings to real-world applications remains a challenge. People react differently to treatments, and side effects can be an issue. These issues can be addressed with additional treatments, as Dr. Wang says, “I would say nanomaterials. In nerve injury and after tumor removal, it is now common to apply pieces of matrix gel to lesion tissue after surgery. It is still a potential way to have formulated gel injected into demyelinating lesion that can protect nerves, alleviate inflammation, and promote oligodendrocyte production.”
