The Evil Twin of Climate Change: Ocean Acidification
A shell dissolving over time as carbonic acid is decalcifying the shell.
Imagine: All marine life on Earth dying because people wanted to make a few more bucks selling crayons or bundles of paper. Well, there is no need to imagine, because each day, as more and more greenhouse gasses are released into the environment through the machinery’s by-products from producing these crayons and paper, marine life is slowly dying off.
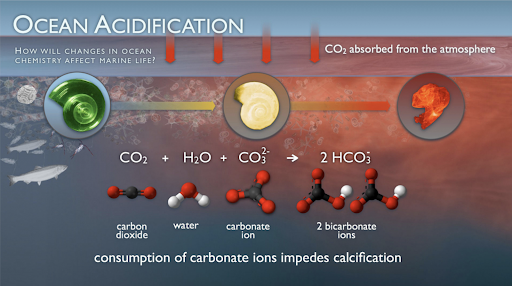
According to the National Oceanic and Atmospheric Association, over the last 200 years, humans have been able to single-handedly decrease the pH level of the 7 oceans by 0.1. The pH scale is a scale from 1 to 14, where 7 is water, a neutral non-acidic and non-basic liquid. The pH level is a measurement of acidity, so a 0.1 difference in the pH, even though it may not seem a lot, can really affect ocean life in a negative way. The acidity (pH) of oceans has risen by 30% as a consequence of carbon dioxide being released into the atmosphere through the process of burning fossil fuels, deforestation, and pollution. Carbon dioxide molecules released into the atmosphere are absorbed by the oceans, and form a bond with water molecules to form an acidic molecule called carbonic acid (H2CO3). The carbonic acid dissociates (splits) into hydrogen ions (H+), and bicarbonate ions (HCO3-). As industries continue to release carbon dioxide into the atmosphere, more CO2 is dissolving in the oceans. As water continues to absorb the CO2 from the atmosphere, more carbonic acid molecules are formed and the ocean’s acidity increases.
How does this contribute to the death of marine life? Well, the acidity in oceans affects coral reefs in the ocean. As the acidity of the ocean increases, the carbonate levels in the ocean decreases. Carbonate is a building block of coral reefs. It helps build the shells that protect the coral reefs. With the decrease in carbonate levels, coral reefs are not able to build their shells, the acidity in the ocean degrades the quality of the shells already protecting the coral reefs. Without this shell, the reef health deteriorates, and the fish and other marine life which rely on it suffer the consequences. Additionally, as the carbonate is being bonded to form carbonic acid, the shells of coral reefs and other marine life are getting dissolved, killing off many marine spies. Lastly, the human communities who rely on fish and other marine life for food and economic growth, suffer.
The amount of carbon dioxide we release in the atmosphere for economic growth will be the death of us if we let it get out of hand. In trying to make a few more dollars trying to sell more boxes of crayons or bundles of paper, we are really just affecting the climate, the ocean, and our own lives.
